Zeroth law of thermodynamics
The Zeroth Law of Thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other.
Thermal equilibrium means that when two bodies are brought into contact with each other and separated by a barrier that is permeable to heat, there will be no transfer of heat from one to the other.
The zeroth law of thermodynamics states that if two systems, A and B, are in thermal equilibrium with a third system, C, then Aand B are in thermal equilibrium with each other.
It is analogous to the transitive property in math (if A=C and B=C, then A=B). Another way of stating the zeroth law is that every object has a certain temperature, and when two objects are in thermal equilibrium, their temperatures are equal.
It is called the "zeroth" law because it came to light after the first and second laws of thermodynamics had already been established and named, but was considered more fundamental and thus was given a lower number — zero.
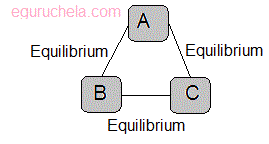
(The Zeroth Law of Thermodynamics)
Other defination of Zeroth law of thermodynamics:
The zeroth law of thermodynamics tells about the concept of temperature to measure hotness or coldness of a body.Two bodies are said to be in thermal equilibrium if no heat transfer takes place when they are in contact.
All bodies in thermal equilibrium are assigned equal temperature.
Zeroth law can be stated as :“ If two bodies lets say A and B are in thermal equilibrium and A and C are also in thermal equilibrium, then B and C are also in thermal equilibrium.”
The temperatures of two bodies decides the direction of heat flow when the two bodies are kept in contact. Heat flows from higher temperature to the body at lower temperature.
What is defination of Thermal Equilibrium ?
Defination of Thermal Equilibrium
Two systems are in thermal equilibrium when there is no net transfer of energy as heat between the two systems, when they are in thermal contact.
At equilibrium, the systems have the same temperature.It can be a "dynamic equilibrium". That means heat is exchanged, but the heat going one way is exactly balanced by heat going in the opposite direction.
Incidentally, the Zeroth law of thermodynamics is about thermal equilibrium.
Example and Applications:
Example of zeroth law of thermodynamics is Mercury thermometer in Mercury thermometer the glass body of thermometer acts as body B, human body A and Mercury acts as body C.
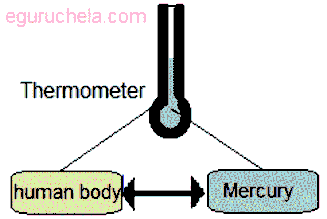
(Example of Zeroth Law of thermodynamics)
