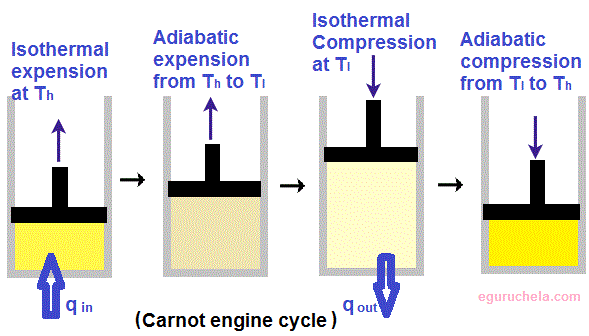
Carnot engine
What is Carnot Engine ?
In 1834 , Benoît Paul Émile Clapeyron graphically expanded the Carnot engine model and mathematically elaborated upon by Rudolf Clausius in 1857 from which the concept of entropy emerged. A Carnot heat engine is an engine that operates on the reversible Carnot cycle.
Every thermodynamic system exists in a particular state. A thermodynamic cycle occurs when a system is taken through a series of different states, and finally returned to its initial state.
In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine.
The most efficient heat engine cycle is the Carnot cycle, consisting of two isothermal processes and two adiabatic processes.
The Carnot cycle can be thought of as the most efficient heat engine cycle allowed by physical laws. When the second law of thermodynamics states that not all the supplied heat in a heat engine can be used to do work, the Carnot efficiency sets the limiting value on the fraction of the heat which can be so used.
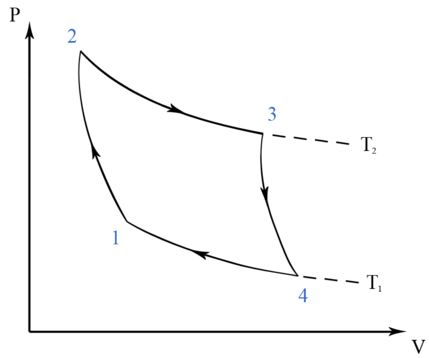
Diagramatic Explanation
Carnot Theorem:
Carnot Theorem states that when two given temperatures be T1 and T2 then system working between can never have an efficiency more than the Carnot engine working between the same reservoirs.Steps involved in a Carnot Cycle :
Step 1 is Isothermal expansion
Step 2 is Adiabatic expansion
Step 3 is Isothermal compression
Step 4 is Adiabatic compression