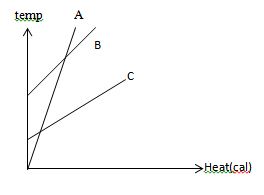
Specific heat capacity
Specific heat capacity is the amount of heat energy required to raise the temperature of a body per unit of mass. he specific heat is the amount of heat per unit mass required to raise thetemperature by one degree Celsius. The relationship between heat and temperature change is usually expressed in the form shown below where c is the specific heat. The relationship does not apply if a phase change is encountered, because the heat added or removed during a phase change does not change the temperature.
Q = cmΔT
Where
A state variable is one of the set of variables that are used to describe the mathematical "state" of a dynamical system. Intuitively, the state of a system describes enough about the system to determine its future behaviour in the absence of any external forces affecting the system. Models that consist of coupled first-order differential equations are said to be in state-variable form.
hermodynamic variables describe the momentary condition of a thermodynamic system. Regardless of the path by which a system goes from one state to another — i.e., the sequence of intermediate states — the total changes in any state variable will be the same. This means that the incremental changes in such variables are exact differentials. Examples of state variables include
