Specific and molar conductivity
Specific conductance is a measure of the electric current in the water sampled carried by the ionized substances, therefore the dissolved solids are basically related to this measure that is also influenced by the good conductivity of inorganic acids, bases and salts the poor conductivity characteristics of organic compounds.
It is the reciprocal of specific resistance.
k = 1/p
(i) Ohm’s Law:
It states the current (I) flowing through a conductor at a given temperature, is proportional to the potential difference (V) and inversely proportional to the resistance (R).
Both the metallic as well as electrolytic conductors obey ohm’s law.
I = V/R Mathematically
(ii) Resistances (R) :
It is the property of a substance by which it obstructs the flow of current through it.
Its SI unit is ohm (038F)
Here,
l / A is fixed for a conductor, called cell constant and ρ(Rho), the constant of proportionality is called the Resistivity or specific resistance.
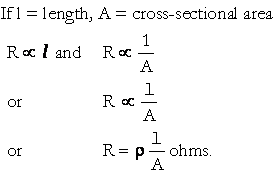
Hence, Resistance = Resistivity _ cell constant.
If, in a uniform conductor, l = 1m, A = 1m2 then R = ρ
Thus, Resistivity of a uniform conductor is equal to its resistance when its length is 1 m and its cross-sectional area is 1 m2.
The SI unit of resistivity is ohm m (038F).
Conductance (C):
It is the reciprocal of the electrical resistance, i.e. C = 1 / R .
It is expressed as 038F−1 or mho and its unit is siemen, S ( = 038F−1).
Conductivity or specific conductance (k) is the reciprocal of resistivity or specific resistance (ρ).
It is the conductance between opposite faces of 1 m cube conductor

Molar conductivity (038F):
The conductivity produced by dissolving 1 gram-mole of an electrolyte placed between two large electrodes at one centimeter apart.
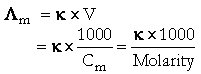
Where, V = Volume of solutions (in cm3) containing one mole of electrolyte
Cm = molar concentration (mol L−1) or morality.
Units of molar conductivity = ohm−1cm−1mol−1
The relationship between molar conductivity and specific conductivity
The Specific Conductivity = (Molar Conductivity) X (Molarity of the solution).
