Redox reactions
An oxidation-reduction reaction is a type of chemical reaction that involves a transfer of electrons between two species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. in general, redox reactions involve the transfer of electrons between chemical species.
The chemical species from which the electron is stripped is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. Oxygen is not necessarily included in such reactions as other chemical species can serve the same function.
The term "redox" comes from two concepts involved with electron transfer: reduction and oxidation. It can be explained in simple terms:

Reduction is the gaining of hydrogen
Therefore, combustion reactions are good examples of redox reactions where one molecule gains oxygen (is oxidized) and one molecule gains hydrogen (is reduced).
For example, let’s look at what happens when gasoline in your car is burned as you drive around town (heptane is a common hydrocarbon component of gasoline).
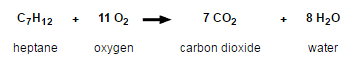
We all intuitively know the chemical reaction of rusting:

