Band theory of solids
A useful way to visualize the difference between conductors,insulators and semiconductors is to plot the available energies for electrons in the materials. Instead of having discrete energies as in the case of free atoms, the available energy states form bands. Crucial to the conduction process is whether or not there are electrons in the conduction band. semiconductors will actually act as insulators at absolute zero. Above this temperature and yet still staying below the melting point of the solid, the metal would act as a semiconductor.Semiconductors are classified by the fully occupied valence band and unoccupied conduction band. With the small band gap in between these two bands, it takes a certain amount of energy to excite the electrons from the valence to conduction band.
The band theory looks at the jump of electrons across the band gap. In particular, the jump of electrons from their valence band to their conduction band across their Fermi energy level. This "jump" dictates optical and magnetic properties of the solid.
Valence Band
The band of energy where all of the valence electrons reside and are involved in the highest energy molecular orbital.
Conduction Band
The band energy where positive or negative mobile charge carriers exist. Negative mobile charge carriers are simply electrons that had enough energy to escape the valence band and jump to the conduction band. Here, they move freely throughout the crystal lattice and are directly involved in the conductivity of semiconductors. Positive mobile charge carriers are also referred to as holes. Holes refer to the lack of an electron in the conduction band.
Fermi Level
This level refers to the highest occupied molecular orbital at absolute zero. It is usually found at the center between the valence and conduction bands. The particles in this state each have their own quantum states and generally do not interact with each other.
Semiconductors
Semiconductors are defined to have conductivity in between an insulator and a conductor. Due to this property, semiconductors are very common in every day electronics since they likely will not short circuit like a conductor. They get their characteristic conductivity from their small band gap. Having a band gap prevents short circuits since the electrons aren't continuously in the conduction band.
Filling of bands
At thermodynamic equilibrium, the likelihood of a state of energy E being filled with an electron is given by the Fermi–Dirac distribution, a thermodynamic distribution that takes into account the Pauli exclusion principle.
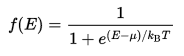
Go to index page
