Types of unit cell
The simplest repeating unit in a crystal is called a unit cell. Each unit cell is defined in terms of lattice points the points in space about which the particles are free to vibrate in a crystal.
Crystal lattice is the depiction of three dimensional arrangements of constituent particles (atoms, molecules, ions) of crystalline solids as points.
Or the geometric arrangement of constituent particles of crystalline solids as point in space is called crystal lattice.
Crystal lattice and Unit cell
Characteristics of crystal lattice
Each constituent particle is represented by one point in a crystal lattice.
These points are known as lattice point or lattice site.
Lattice points in a crystal lattice are joined together by straight lines.
By joining the lattice points with straight lines the geometry of the crystal lattice is formed.

Parameters of a unit cell:
A unit cell is characterized by six parameters. These parameters are three edges (a, b and c) and angles between them .
Dimensions along the edges of a unit cell is represented by a, b and c.
Edges of unit cell may or may not be mutually perpendicular.
The angle between b and c is represented by α, between a and c by β and between a and b by γ.
Types of Unit Cell
There are two types of unit cells – Primitive and Centred Unit Cells.
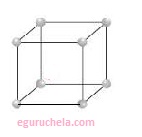
Primitive Unit Cells:
When particles in unit cell are present only at the corners,
it is called the primitive unit cell or the unit cell constructed so that it contains only one lattice point is called primitive cell means that there is n number of cells sharing the lattice point,
each vertex of the cell sits on a lattice point which is shared with the surrounding cells and each lattice point is said to contribute 1/n to the total number of lattice points in the cell.
Centred Unit Cells:
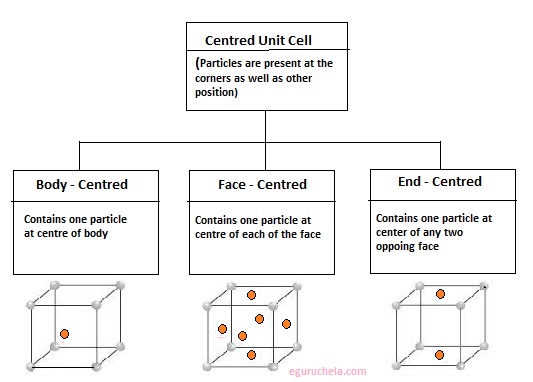
When particles are present at other positions in addition to those at corners in a unit cell, it is called a Centred Unit Cell.
Go to index page
