Lequid crystals
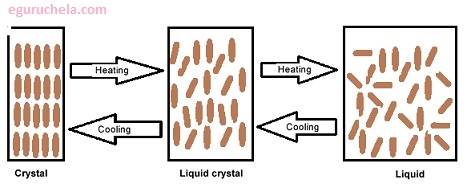
In a crystalline solid, the molecules are well ordered in a crystal lattice . When a crystal is heated, the thermal motions of the molecules within the lattice become more vigorous, and eventually the vibrations become so strong that the crystal lattice breaks down and the molecules assume a disordered liquid state. The temperature at which this process occurs is the melting point .
Liquid crystals can be classified into two main categories: thermotropic liquid crystals, and lyotropic liquid crystals.
Thermotropic transactions occur in most liquid crystals, and they are defined by the fact that the transitions to the liquid crystalline state are induced thermally. That is, one can arrive at the liquid crystalline state by raising the temperature of a solid and/or lowering the temperature of a liquid.
Thermotropic liquid crystals can be classified into two types: enantiotropic liquid crystals, which can be changed into the liquid crystal state from either lowering the temperature of a liquid or raising of the temperature of a solid, and monotropic liquid crystals, which can only be changed into the liquid crystal state from either an increase in the temperature of a solid or a decrease in the temperature of a liquid, but not both.
In a crystalline solid, the molecules are well ordered in a crystal lattice . When a crystal is heated, the thermal motions of the molecules within the lattice become more vigorous, and eventually the vibrations become so strong that the crystal lattice breaks down and the molecules assume a disordered liquid state. The temperature at which this process occurs is the melting point . Although the transition from a fully ordered structure to a fully disordered one takes place in one step for most compounds.
Design of liquid crystalline materials
A large number of chemical compounds are known to exhibit one or several liquid crystalline phases. Despite significant differences in chemical composition, these molecules have some common features in chemical and physical properties. There are three types of thermotropic liquid crystals: discotics, bowlics and rod-shaped molecules.
The molecular shape should be relatively thin, flat or bowl-like, especially within rigid molecular frameworks.
• The molecular length should be at least 1.3 nm, consistent with the presence of long alkyl group on many room-temperature liquid crystals.
• The structure should not be branched or angular, except for the bowlics.
• A low melting point is preferable in order to avoid metastable, monotropic liquid crystalline phases.
