Resonance
Theory by which the actual normal state of a molecule is represented not by a single valence-bond structure but by a combination of several alternative distinct structures. The molecule is then said to resonate among the several valence-bond structures or to have a structure that is a resonance hybrid of these structures.
The energy calculated for a resonance hybrid is lower than the energies of any of the alternative structures; the molecule is then said to be stabilized byresonance. The difference between the energies of any one of the alternative structures and the energy of the resonance hybrid is designated resonance energy.
The classic example of the application of the theory of resonance is the formulation of the structure ofbenzene. The structure of benzene as a six-membered ring of carbon atoms was introduced by the German chemist F.A. Kekule in 1865.
To make the structure compatible with the quadrivalence of carbon, he introduced alternating single and double bonds in the ring, and in 1872, in order to account for the fact that no isomers of benzene (no isomeric orthosubstituted benzenes differing in having single or double bonds between the substituted carbon atoms) had been observed, he introduced the idea of an oscillation between structures of the form:
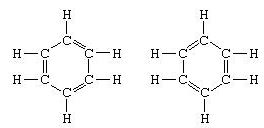
The concept of resonance has similarly been used to formulate structures for polynuclear aromatic hydrocarbons, molecules containing conjugated systems of double bonds free radicals, and other molecules to which no satisfactory single structure in terms of single bonds, double bonds, and triple bonds can be assigned. Some general rules are used in the selection of suitable resonance structures for a molecule. These rules are: the structures must have energies of similar magnitudes; the arrangement of the atoms must be approximately the same in all the structures; and the structures must have the same numbers of unpaired electrons.
Misconception
It is a common misconception that resonance structures are actual transient states of the molecule, with the molecule oscillating between them or existing as an equilibrium between them. However these individual contributors cannot be observed in the actual resonance-stabilized molecule. Any molecule or ion exists in only one form – the resonance hybrid.
Due to confusion with the physical meaning of the word resonance, as no elements actually appear to be resonating, it has been suggested that the term resonance be abandoned in favor of delocalization.
Resonance energy would thus become delocalization energy and a resonance structure becomes a contributing structure. The double headed arrows would be replaced by commas to illustrate a set of structures rather than suggesting that there is a reaction that converts among them.
Rules for estimating stability of resonance structures
1. The greater the number of covalent bonds, the greater the stability since more atoms will have complete octets
2. The structure with the least number of formal charges is more stable
3. The structure with the least separation of formal charge is more stable
4. A structure with a negative charge on the more electronegative atom will be more stable
5. Positive charges on the least electronegative atom (most electropositive) is more stable
6. Resonance forms that are equivalent have no difference in stability and contribute equally
