Hydrogen Bond
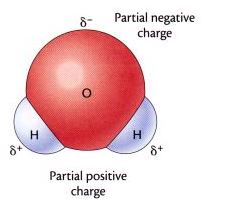
A hydrogen bond is a type of attractive (dipole-dipole) interaction between an electronegativeatom and a hydrogen atom bonded to another electronegative atom. This bond always involves a hydrogen atom. Hydrogen bonds can occur between molecules or within parts of a single molecule. A hydrogen bond tends to be stronger than van der Waals forces, but weaker than covalent bonds or ionic bonds.
The hydrogen bond is a wondrous thing. It helps give snowflakes their hexagonal symmetry; binds DNA into a double helix; shapes the three-dimensional forms of proteins; and even raises water’s boiling point high enough to make a decent cup of tea. A chemical bond in which a hydrogen atom of one molecule is attracted to an electronegative atom, especially anitrogen, oxygen, or fluorine atom, usually of another molecule.
Hydrogen bonds can vary in strength from very weak (1–2 kJ mol−1) to extremely strong (161.5 kJ mol−1 in the ion HF−
2) Typical enthalpies in vapor include
• F−H…:F (161.5 kJ/mol or 38.6 kcal/mol)
• O−H…:N (29 kJ/mol or 6.9 kcal/mol)
• O−H…:O (21 kJ/mol or 5.0 kcal/mol)
• N−H…:N (13 kJ/mol or 3.1 kcal/mol)
• N−H…:O (8 kJ/mol or 1.9 kcal/mol)
• HO−H…:OH(18 kJ/mol or 4.3 kcal/mol) data obtained using molecular dynamics as detailed in the reference and should be compared to 7.9 kJ/mol for bulk water, obtained using the same molecular dynamics.)
Bifurcated and over-coordinated hydrogen bonds in water
A single hydrogen atom can participate in two hydrogen bonds, rather than one. This type of bonding is called "bifurcated". It can exist, for instance, in complex natural or synthetic organic molecules. It has been suggested that a bifurcated hydrogen atom is an essential step in water reorientation. Acceptor-type hydrogen bonds (terminating on an oxygen's lone pairs) are more likely to form bifurcation than are donor-type hydrogen bonds, beginning on the same oxygen's hydrogens.