Electrophiles and nucleophiles
Electrophiles
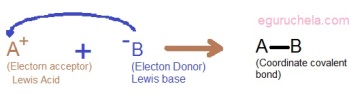
The word electrophile can be split into “electro” derived from electron and “phile” which means loving. The electrophiles are positively charged or neutrally charged, the electrophiles are deficient in electrons and can accept a couple of electrons, it also called as Lewis acid.
Because of electron deficiency they are electrons loving and attract electrons, The movement of electrons depends on the density. The movement is from high-density area to low density area. They undergo electrophilic addition and electrophilic substitution reactions.
Nucleophile
A nucleophile is a chemical species that donates an electron pair to an electrophile to form a chemical bond in relation to a reaction. All molecules or ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.
Nucleophilic describes the affinity of a nucleophile to the nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms.
Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge.
Difference between Electrophile and Nucleophile
| Electrophile | Nucleophile | ||
|---|---|---|---|
| Called as | Lewis acid | Lewis base | |
| Charge | Positively charged / neutral | Negatively charged / neutral | |
| Type of reaction | Show electrophilic addition and electrophilic substitution reactions | Show nucleophilic addition and nucleophilic substitution reactions | |
| Presence of electron | Electron-poor | Electron-rich | |
| Movement of electrons | Accepts a pair of an electron to form a covalent bond | Donates a pair of an electron to form a covalent bond | |
| Example | Hydronium Ion | Chloride Ion |
