Electrometric effect
Electromeric effect refers to a molecular polarizability effect occurring by an intramolecular electron displacement (sometimes called the ‘conjugative mechanism’ and, previously, the ‘tautomeric mechanism’) characterized by the substitution of one electron pair for another within the same atomic octet of electrons. However, this term is now considered and this effect is considered along with the inductive effect.
If the attacking species is an electrophile, the π electrons are transferred towards the positively charged atom. This is the +E effect.
Types of Electromeric Effects
The electromeric effect can be either the +E effect or the -E effect. This classification is done based on the direction in which the electron pair is transferred.
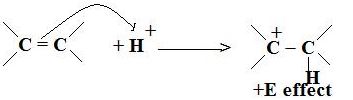
+E Effect
In +E effect, the electron pair of the pi bond is moved towards the attacking reagent. It can be observed in the addition of acid to alkenes. The attacking reagent attaches itself to the atom which obtained an electron pair in the transfer means the π electrons are transferred towards the positively charged atom.
-E Effect
In -E effect the electron pair of the pi bond is moved away from the attacking reagent. The attacking reagent attaches itself to the positively charged atom in the molecule..Difference between inductive effect and Electromeric effect
The inductive effect is observed when two atoms with different electronegativity values form the chemical bond in otherhand the electromeric effect occurs when a molecule having multiple bonds is exposed to an attacking agent such as a proton.
The inductive effect is a permanent effect and irreversible in otherhand electromeric effect is a temporary effect and is reversible
