Cells, emf and Internal Resistance
The cell consists of electrodes and electrolytes and generates the electricity by using chemical energy. The cell is consist of electrodes and electrolytes, Electrodes can be in a form of wire or rod or plates which are conductors through which current can pass. The electrolyte is a substance where free ions are present and which can conduct electricity.
The ZnSO4and CuSO4 is used as electrolyte in galvanic cell. The Zn has tendency to become Zn ion, meams to lose 2 electrons present in the valence shell (Zn-> 2e- + Zn2+)
The Cu ion has the tendency to gain electron and become Cu and stick to electrode (Cu2++2e- -> Cu)
When Zn and Cu are joined together with a conducting wire than electrons will flow from Zn to Cu.
Electrodes of a Cell: The battery has two terminals (Positive and Negative). The Positive Terminal is called Cathode and the Negative Terminal is called Anode. The electrodes will be dipped in a solution called electrolyte.
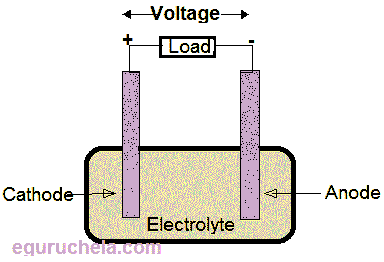
(Components of a cell)
The voltage of a cell depends upon a many factors including material of electrodes (made from) and the substance used as the electrolyte .
When current flows round a circuit energy is transformed in both the external resistor but also in the cell itself.
All cells have a resistance of their own and we call this the internal resistance of the cell.
The voltage produced by the cell is called the electromotive force or e.m.f for short and this produces a p.d across the cell and across the external resistor.
the electromotive force or e.m.f. is the energy provided by a cell or battery per coulomb of charge passing through it, it is measured in volts (V). It is equal to the potential difference across the terminals of the cell when no current is flowing.
ε = E/Q
- ε= electromotive force in volts, V
- E = energy in joules, J
- Q = charge in coulombs, C
ε = I(R + r)
ε = electromotive force in volts, V
I = current in amperes, A
R = resistance of the load in the circuit in Ω
r = internal resistance of the cell in Ω
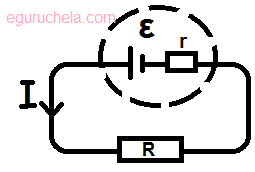
(Electromotive Force and Internal Resistance)
The graph of terminal p.d. against current
The graph of terminal potential difference (V) against the current in the circuit (I) can be plotted which is a straight line with a negative gradient.
Now rearrange the e.m.f. equation to match the general experesion for a straight line: y = mx +c.
ε = V + Ir
ε - Ir = V
V = -rI + ε
y = mx + c (line equation)
This proves, the e.m.f. of the cell intercept on the y-axis and gradient of the graph is equal to -r (internal resistance of the cell).
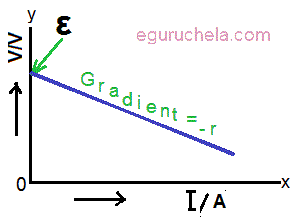
(Graph (terminal p.d. against current))
The factors affecting electrode potential
The concentration of ions in the solution.
The temperature of the system.
The chemical nature of the metal or nonmetal.
The number of electrons transferred in the half cell reactions.
