Coulombs Law
Coulomb's Law is a law of physics that describes the interaction between electrically charged objects.
It was first defined by physicist Charles-Augustin de Coulomb in 1783.
This law states that the electrostatic force between two objects is proportional to the product of the charge of each of the objects and
inversely proportional to the square of the distance between these two objects.
Specifically, F = Ke (q1q2/r2) ,
where F is the electrostatic force,
Ke represents a constant value,
q1 represents the charge of the first object,
q2 represents the charge of the second object and
r represents the distance between the two charges.
The constant value ke or k is dependent on the medium in which the two objects reside.
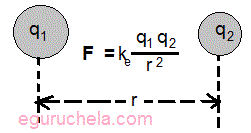
Coulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles.
The law was first published in 1784 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism.
It is analogous to Isaac Newton's inverse-square law of universal gravitation.
Coulomb's law states that:
The magnitude of the electrostatic force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distance between them.
This shows, the inverse square dependence of the electric force. It can be used to provide a relatively simple derivative of Gauss's law for general cases. Finally, the vector form of Coulomb's law is important because it helps us to specify the direction of electric fields due to charges.
Coulomb's Law Constant Value
Determine the value of k:
k = 1/4πϵ0
We know that ϵ0 (value of the dielectric constant or the electric permittivity at free space) = 8.85 x 10⁻¹² C²/Nm².
Apply the value of ϵ0
k = 1/4π × 8.85×10−12(C2/Nm2)
k = 1/(4π × 8.85×10−12)Nm2C-2
k = 8.99 x 109Nm2C-2
Application Of The Coulombs Law
Calculating the distance and force between two charges.
Calculating the force at a point due to the presence of multiple points (theorem of superposition).
The electric field can be calculated using Coulomb's law.
Limitations of Coulomb's Law
It is difficult to apply Coulomb's law where the charges are of arbitrary shape because in such cases we cannot determine the distance between the charges.
Coulomb's Law cannot be directly used to calculate the charge on large planets.
Coulomb's Law is only applicable for point charges at rest.
Coulomb's law can be applied only in cases where the inverse square law is observed.
