Factors affecting adsorption of gases on solids
When a solid surface is brought in contact with a gas or liquid, molecules from the gas or liquid starts to collect at the surface of the solid.
This phenomenon of collection of gas or liquid molecules on the surface of the solid is known as adsorption. A substance which accumulates on the solid surface is known as adsorbate and the solid surface on which it occurs is known as adsorbent.
Types of Adsorption
There are two types of adsorption as follows:
1. Physical adsorption or physisorption
The gas molecules accumulate on the surface of solids via weak van der Waal forces. This process is non-specific and reversible. The physisorption occures at low temperatures and chemical adsorption begins when temperature rises.
2. Chemical adsorption or chemisorption
The Gas molecules accumulate on the surface of solids via ionic or covalent bonds. Because chemical bonds are involved therefore process is highly specific, irreversible in nature, and requires high activation energy.
Following are the factors which influence the adsorption of gases by solids
1. Surface area
2. Nature of gas
3. Temperature
4. Pressure
1. Surface Area
Adsorption being a surface phenomenon, the extent of adsorption depends upon the surface area. Increase in the surface area of the adsorbent, increases the total amount of gas adsorbed. Thus finely divided metals (nickel, platinum) and porous substances (Charcoal, silica gel) provides large surface area and are best solid adsorbents.
2.Nature of Gas
The amount of gas adsorbed by a solid depends upon the nature of gas. In general, more easily liquefiable a gas is the more readily will it be adsorbed. Thus 1gm of activated charcoal adsorbs 380 ml of sulphur dioxide 16 ml of methane and 4.5 ml of hydrogen . This is valid for physical adsorption only.
3. Effect of temperature
As adsorption is accompanied by evolution of heat, so according to the Le-Chatelier’s principle, the magnitude of adsorption should decrease with rise in temperature.
4. Surface area of Adsorbent
Larger the surface area of adsorbent more will be active centers and faster will be the rate of adsorption.
Examples of adsorption
- The ammonia or clorine gas is confined in a closed vessale with powdered charcoal the pressure inside the vessal get decrease, it happens because charcoal surface adsorbs the gas mokecules.
- Use of silica get to dry the air, it happens because silica surface adsorbs the water molecules present in the air.
Differentiation Adsorption between Absorption
The adsorption can be differentiated from absorption based on followings:1. Definition:
Adsorption is the loose adherence of gases, liquids, or dissolved solids onto the surface of another solid or liquid.
In case of absorption atoms, molecules or ions enters in another solid or liquid material.
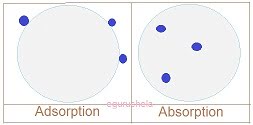
Differentiation Adsorption between Absorption
Nature:
While adsorption is a surface phenomenon where as absorption is a bulk process.
Reaction rate:
The rate of adsorption increases until equilibrium is reached where as absorption occurs at a uniform rate.
Heat exchange:
The adsorption is exothermic used in air conditioners, water purifiers, chillers, etc. where as absorption is endothermic process used in refrigerants, ice production, cold storage, etc.
Concentration:
Concentration of the adsorbed substance changes within the medium where as concentration of absorbed substances remains constant throughout the medium.
