Techniques for water softening
The calcium and magnesium ions in the water are replaced with sodium ions. Since sodium does not precipitate out in pipes or react badly with soap, both of the problems of hard water are eliminated. To do the ion replacement, the water in the house runs through a bed of ion exchange resin. The resins are covered with sodium ions. As the water flows past the sodium ions, they swap places with the calcium and magnesium ions. Eventually, the resin contains nothing but calcium and magnesium and no sodium, and at this point they stop softening the water. It is then time to regenerate the ion exchange resin. Regeneration involves soaking the resin in a stream of sodium ions.
Advantages of Soft Water
1. Soft water has many benefits, prevents Hair loss and Skin infections. Enjoy the luxury of Soft Water while bathing. Also save Money on soap, shampoo and other cleaning materials.
2. Keeps your Clothes Soft and Longer Lasting.
3. Heating appliances like geysers don’t get clogged up by calcium deposits. You will also save Money on Electricity as soft water makes Geysers and other heating appliances at least 20 % more efficient.
Water softening methods
Ion-exchange resin devices
Conventional water-softening appliances intended for household use depend on an ion-exchange resin in which "hardness ions" - mainly Ca2+ and Mg2+ - are exchanged for sodium ions.
Types of ion-exchange materials
Ion exchange resins are organic polymers containing anionic functional groups to which the divalent cations (Ca++) bind more strongly than monovalent cations (Na+). Inorganic materials called zeolites also exhibit ion-exchange properties.
Lime softening
Lime softening is the process in which lime is added to hard water to make it softer.By adding slaked lime [Ca (OH) 2] to hard water, insoluble carbonates are formed. The insoluble calcium carbonate is the scale formed in kettles, boilers, pipes.
Distillation and rain water
Distillation process removes the Ca2+ and Mg2+ which exist in water as nonvolatile salts. The distillation process is too expensive in most cases. The rainwater is soft because it is naturally distilled during the water cycle of evaporation, condensation and precipitation.
Boiling the water
On boiling, the soluble bicarbonate is decomposed into insoluble carbonate.
Chelating agents
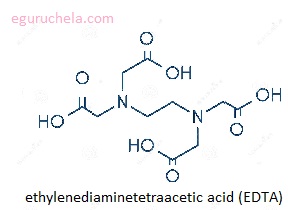
The chelators are used in chemical analysis as water softeners and chelators are ingredients in many commercial products such as shampoos and food preservatives. Citric acid is used to soften water in soaps and laundry detergents. The most commonly used synthetic chelator is ethylenediaminetetraacetic acid (EDTA).
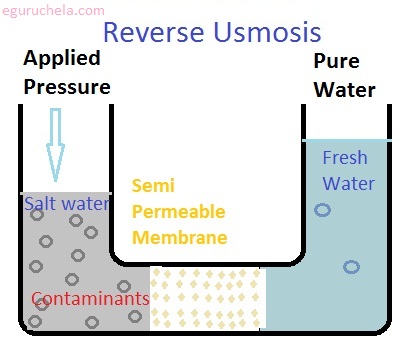
Reverse osmosis
It is the major technology for water softening and deployed on a large scale. The reverse osmosis systems incorporate pre and post filters along with the membrane itself. As per description of NSF/ANSI Standard 44, it reverses by the application of pressure, the flow of water in a natural process of osmosis so that water passes from a more concentrated solution to a more dilute solution through a semi-permeable membrane.
Go to index page
