Hardness of water
The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water.
Hard drinking water may have moderate health benefits, but can pose serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling towers, and other equipment that handles water.
Hard water is high in dissolved minerals, both calcium and magnesium.
We may have felt the effects of hard water, literally, the last time you washed your hands.
Depending on the hardness of your water, after using soap to wash you may have felt like there was a film of residue left on your hands.
The water hardness got some benefits also. The hard drinking water contributes a small amount toward total calcium and magnesium needs for human diet.
Humans need minerals to stay healthy as per the National Research Council (National Academy of Sciences).
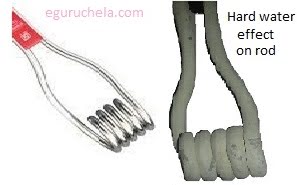
When hard water is heated in a home water heater, solid deposits of calcium carbonate can form which can reduce the life of equipment, raise the costs of heating the water, lower the efficiency of electric water heaters and clog pipes.
Measures of water hardness
Mineral deposits are formed by ionic reactions resulting in the formation of an insoluble precipitate. For example, when hard water is heated, Ca2+ ions react with bicarbonate (HCO3-) ions to form insoluble calcium carbonate (CaCO3).
Ca2+(aq) + 2HCO3-(aq)------>CaCO3(s)+H2O
The term "hardness" comes from the fact that it is hard to get soapsuds from soap or detergents in hard water. This happens because calcium and magnesium react strongly with negatively-charged chemicals like soap to form insoluble compounds.
Water hardness is of two types
(1) temporary hardness is caused by dissolved calcium bicarbonate (Ca(HCO3)2) and can be cured by boiling or by adding calcium hydroxide or lime (Ca(OH)2) to water.
(2) permanent hardness is caused by dissolved calcium sulphate (CaSO4) and cannot be cured by boiling, but by certain processes such as 'ion exchange' using polyphosphates or zeolites .
The classification depends on organizations to organizations which may slightly different, the U.S. Department of Interior and the Water Quality Association classified the water hardness as follows:| Classification | mg/l or ppm | grains/gal |
| Soft | 0 - 17.1 | 0 - 1 |
| Slightly hard | 17.1 - 60 | 1 - 3.5 |
| Moderately hard | 60 - 120 | 3.5 - 7.0 |
| Hard | 120 - 180 | 7.0 - 10.5 |
| Very Hard | 180 & over | 10.5 & over |
Disadvantages of Hard Water:
It is very difficult to wash clothes with hard water as it requires more soap . When hard water is boiled at home or in industries, it leaves deposits of calcium and magnesium salts in kettles, hot-water pipes, boilers and radiators. These deposits reduce the efficiency of boilers, kettles and pipes and can cause blockages and even bursting of the boilers.
Types of Hardness
Based on the behavior of water towards soap, hardness is divided into two types as follows:1. Temporary hardness:
If the hardness is due to the presence of soluble bicarbonates of calcium and Magnesium is called temporary hardness. When water containing dissolved carbon dioxide passes over solid then these compounds get dissolved in water. Rainwater and distilled water are always soft because they do not have soluble salts.2. Permanent hardness
Permanent hardness is due to the presence of chlorides and sulphates of calcium and magnesium. the Permanent hardness can be removed by the addition of washing soda. This removes both the temporary and the permanent hardness of water.Go to index page
