Diazonium salts: Preparation
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R−N+2X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halogen. Diazonium salts, especially those where R is an aryl group, are important intermediates in the organic synthesis of azo dyes.
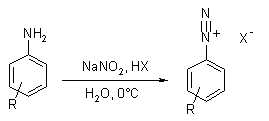
The nitrosation of primary aromatic amines with nitrous acid leads to diazonium salts, which can be isolated if the counterion is non-nucleophilic.
Diazonium salts are important intermediates for the preparation of, and azo compounds. Diazonium salts can react as pseudohalide-type electrophiles, and can therefore be used in specific protocols for the Heck Reaction or Suzuki Coupling.
The diazonium group can be replaced by numerous atoms or groups of atoms, often with the aid of copper or a copper salt; these reactions make possible the preparation of a wide variety of aromatic derivatives. Chemical reduction of aromatic diazonium salts leads to formation of hydrazine derivatives.
