Methods of preparation
Amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, where in one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Many organic halogen compounds are converted into amines by treatment with aqueous (or) alcoholic solution of ammonia.
This reaction is generally carried out either by allowing the reactants to stand together at room temperature (or) by heating them under pressure. Displacement of halogen by NH3 yields the amine salt, from which free amine can be liberated with hydroxide ion.
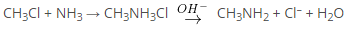
Amines are organic compounds which contain and are often actually based on one or more atoms of nitrogen. Structurally amines resemble ammonia in that the nitrogen can bond up to three hydrogens, but amines also have additional properties based on their carbon connectivity. In an amine, one or more of the hydrogen atoms from ammonia are replaced by organic substituents like alkyl (alkane chain) and aryl (aromatic ring) groups.
