Davisson-Germer experiment
In 1927 scientists C.J. Davisson and L.H. Germer carried out an experiment (using electron diffraction method) to explain an electron's wave nature, The Davisson-Germer experiment demonstrated the wave nature of the electron, confirming the earlier hypothesis of deBroglie.
Putting wave-particle duality on a firm experimental footing, it represented a major step forward in the development of quantum mechanics.
The experiment not only played a major role in verifying the de Broglie hypothesis and demonstrated the wave–particle duality, but also was an important historical development in the establishment of quantum mechanics and of the Schrödinger equation.
Louis de Broglie presented his thesis concerning the wave–particle duality theory, which proposed the idea that all matter displays the wave–particle duality of photons. According to de Broglie, for all matter and for radiation alike, the energy E of the particle was related to the frequency of its associated wave v by the Planck relation:
E = h /λ
And that the momentum of the particle p was related to its wavelength by what is now known as the de Broglie relation:
P = h / λ
where h is Planck's constant.
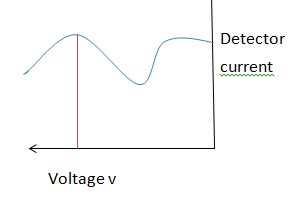
Davisson-Germer electron diffraction experiment observation
Davisson Germer electron diffraction experiment indicates that a detector receives electronic current ( means presence of an electron in a particle form).
Therefore intensity of an electric current produced or received and scattered in an angle.
This current referred to as electron intensity.
The intensity of a scattered electron is never continuous.
The maximum and minimum value of a diffraction pattern can be found by studying potential differences and scattering angles.
