Calorimetry
Calorimetry is the science or act of measuring changes in state variables of a body for the purpose of deriving the heat transferassociated with changes of its state due for example to chemical reactions, physical changes, or phase transitions under specified constraints.
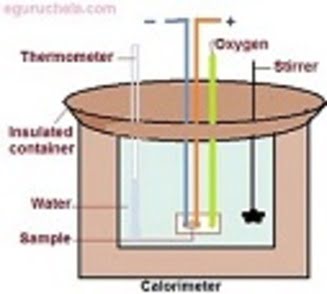
Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat and the Greek word μέτρον (metron), meaning measure.
Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry
Calorimetry, derived from the Latin calor meaning heat, and the Greek metry meaning to measure, is the science of measuring the amount of heat.
All calorimetric techniques are therefore based on the measurement of heat that may be generated (exothermic process), consumed (endothermic process) or simply dissipated by a sample.
There are numerous methods to measure such heat, and since calorimetry's advent in the late 18th century, a large number of techniques have been developed.
Initially techniques were based on simple thermometric (temperature measurement) methods, but more recently, advances in electronics and control have added a new dimension to calorimetry, enabling users to collect data and maintain samples under conditions that were previously not possible.
The principle of calorimetry
The principle of calorimetry indicates the law of conservation of energy means the total heat lost by the hot object is equal to the total heat gained by the cold object.
However, heat flow continues until a state of thermal equilibrium is achieved between the objects
The heat transfer in a system is calculated using the formula:
q = mcΔt
Where
q = measure of heat transfer
m = mass of the body
c = specific heat of the body
Δt = change in the temperature
Example
The temperature of a calorimeter increases 0.10 K when 7.52 J of electric energy is used to heat it. What is the heat capacity of the calorimeter?
Dividing the amount of energy by the temperature increase yields the heat capacity, C,
C = 7.52 / 0.10 = 75.2 J/K.
We often compare the heat capacity of a calorimeter to that of a definite amount of water.
The heat capacity of 75.2 J/K for the calorimeter is equivalent to the heat capacity of 1 mole (18 g) of water (18 g mol-1*1 cal (g K)-1*4.184 J cal-1 = 75.3 J (K mol)-1.
electric energy = q * dV = i * dt * dV, where q is charge; dV, voltage; i, current; and t, time.
Measuring the enthalpy by Calorimeter
By definition, dH is the energy (heat) released at constant pressure, whereas dE is the energy released at constant volume. These two quantities are related by the equation.
dH = dE + (d(P V))
dH = dE + (d(P V))
where dn = number of moles of gas in the products - number of moles of gas in the reactants.
The Bomb Calorimeter
For combustion reactions, we often enclose all reactants in an explosive-proof steel container, called the bomb whose volume does not change during a reaction.
The bomb is then submerged in water or other liquid that absorbs the heat of reaction.
The heat capacitor of the bomb plus other things is then measured using the same technique as other calorimeters.
Such an instrument is called a bomb calorimeter, and its application is called the bomb calorimetry.
qv = C * dT,
where ,
dT is the temperature increase.
The qv so measured is also called the change in internal energy.
Use of calorimetry
Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. This requires careful measurement of the temperature change that occurs during the process and the masses of the system and surroundings.
Example :
When a 10.0-watt (J/s) heater is used to heat a bomb calorimeter, its temperature increases by 3.0 K in 5.0 min. Calculate the heat capacity of the calorimeter.
Energy used = 10 * 5 * 60 = 3000 J.
Thus, C = 3000 / 3.0 = 1000 J/K,
This is equivalent to (1000 J/K) / (75.2 J/(K mol))
= 13.3 moles of water
