Atomic Structure
Atom
The smallest possible amount of matter which still retains its identity as a chemical element, consisting of a nucleus surrounded by electrons.
Atomic Particles
Atoms are made up of three particles called as protons, neutrons, and electrons. These particles are responsible for the mass and charge of any atoms.
The nucleus is the center of the atom contains the protons having (+) charge and the neutrons have no charge.
The outermost regions of the atom are called electron shells and contain the electrons having (-) charge.
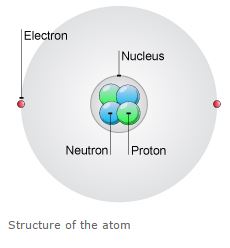
Atoms have different properties based on the arrangement and number of their basic particles.
Proton: Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an element.
Neutron: A subatomic particle forming part of the nucleus of an atom. It has no charge. It is equal in mass to a proton.
In May 1932 James Chadwick announced that the core also contained a new uncharged particle which he named as neutron
Atomic structure details
| Particles | location | Mass (amu) | Charge | Discoverer by | Year of Discovery | Symbol |
|---|---|---|---|---|---|---|
| Proton | Nucleus | 1 (amu) | +1 | E. Rutherford | 1909 | p+ |
| Neutron | Nucleus | 1 (amu) | 0 | James Chadwick | 1932 | no |
| Electron | Orbitals | 0 (amu) | -1 | J.J. Thomson | 1897 | e- |
Related topic : Discovery of Electron , Proton and neutron and Atomic number
Formulas : Structure of atom and Basic concept of chemistry
