Proton and neutron
A neutron is a subatomic particle found in the nucleus of every atom except that of simple hydrogen. The particle derives its name from the fact that it has no electrical charge; it is neutral. Neutrons are extremely dense. If isolated, a single neutron would have a mass of only 1.675 ? 10-27 kilogram, but if a teaspoonful of tightly packed neutrons could be scooped up, the resulting chunk of matter would weigh millions of tons at the earth's surface.
The number of proton in an element's nucleus is called the atomic number. This number gives each element its unique identity. In the atoms of any particular element, for example carbon, the number of protons in the nuclei is always the same, but the number of neutrons can vary.
An atom of a given element having a specific number of neutrons in the nucleus is called an isotope. The isotope of an atom is denoted by writing the element's name followed by the sum of the number of protons and neutrons. The nucleus of a carbon atom always has six protons and usually has six neutrons, but some carbon nuclei contain eight neutrons. Thus, carbon-12 is the most common isotope of carbon; carbon-14 is also found, but is less common.
Neutrons need not be confined to the nuclei of atoms. They can exist all by themselves. When neutrons are found outside atomic nuclei, they acquire fascinating, bizarre, and potentially dangerous properties. When they travel at high speed, they produce deadly radiation. The so-called neutron bomb, known for its ability to kill people and animals while having a minimal effect on inanimate physical structures, works by producing a barrage of high-speed neutrons.
The high density of these particles, combined with their speed, gives them extreme energy. As a result, they have the power to alter, or even break apart, the nuclei of atoms that they strike.
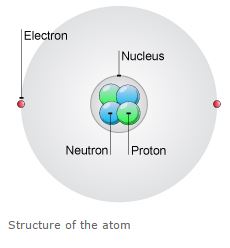
• Atoms are made of extremely tiny particles called protons, neutrons, and electrons.
• Protons and neutrons are in the center of the atom, making up the nucleus.
• Electrons surround the nucleus.
• Protons have a positive charge.
• Electrons have a negative charge.
• The charge on the proton and electron are exactly the same size but opposite.
• Neutrons have no charge.
• Since opposite charges attract, protons and electrons attract each other.
