Werner's theory
Alfred Wernera Swiss chemist put forward a theory to explain the formation of complex compounds. It was the first successful explanation,became famous as the coordination theory of complex compounds, which is also known as Werner's theory.
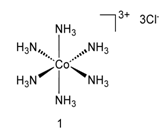
(a) The central metal atom (or) ion in a coordination compound exhibits two types of valencies - primary and secondary.
(1) Primary valencies are ionisable and correspond to the number of charges on the complex ion. Primary valencies apply equally well to simple salts and to complexes and are satisfied by negative ions.
(2)Secondary Valencies are those which a metal cation exercises towards a neutral molecule or negative group in the formation of its complex ions. Thus, secondary valencies may be satisfied by negative ions, neutral molecules having lone electron pair (e.g., H2O, NH3, etc.) or even sometimes by some positive groups. In every case, the coordination number of the metal must be fulfilled.