Mechanism of dehydration
The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
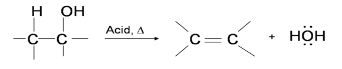
Dehydration reaction of alcohols can be seen as,
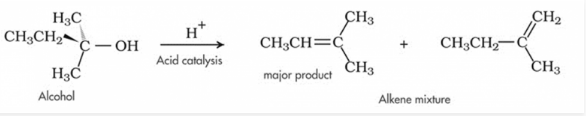
Alcohol as a Base
They can act both as acid or base. The lone pair of electrons on oxygen atom makes the –OH group weakly basic. Oxygen can donate two electrons to an electron-deficient proton. Thus, in the presence of a strong acid, R—OH acts as a base and protonates into the very acidic alkyloxonium ion +OH2 .This basic characteristic of alcohol is essential for its dehydration reaction with an acid to form alkenes.
