Types of Polarization
The charges in a dielectric material won't experience bulk motion in the presence of an external electric field, but they will rearrange themselves in a more subtle manner. This process is known as polarisation and a dielectric material so stressed is said to be polarized. There are two principal methods by which a dielectric can be polarized: stretching and rotation.Electronic polarization is a result of dipole moments induced by the electric field. Atoms are composed of heavy nuclei surrounded by negative electron clouds. In the absence of an external field the movements of the electrons are such that the resultant orbital paths are symmetrical about the nucleus as shown schematically as follows:
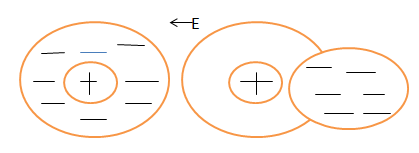
This can be reduced to a simple dipole using the superposition principle. A dipole is characterized by its dipole moment, a vector quantity shown in the figure as the blue arrow labeled M. It is the relationship between the electric field and the dipole moment that gives rise to the behavior of the dielectric.
There are essentially four basic kinds of polarization mechanisms:
Interface polarization
Surfaces, grain boundaries, interphase boundaries may be charged.
Electronic polarization
It also called atom or atomic polarization. An electrical field will always displace the center of charge of the electrons with respect to the nucleus and thus induce a dipole moment as discussed before.
Ionic polarization
In this case a material must have some ionic character. It then automatically has internal dipoles, but these built-in dipoles exactly cancel each other and are unable to rotate. The external field then induces net dipoles by slightly displacing the ions from their rest position.
Orientation polarization
Here the material must have natural dipoles which can rotate freely. In thermal equilibrium, the dipoles will be randomly oriented and thus carry no net polarization. The external field aligns these dipoles to some extent and thus induces a polarization of the material.
