Alpha-particle Scattering and Rutherford’s Nuclear Model of Atom
This is an historic experiment which is often presented to students with little context. It is suggested that, in 1910, the ‘plum pudding model’ was suddenly overturned by Rutherford’s experiment.
In fact, Rutherford had already formulated the nuclear model of the atom before the experiment was carried out; his model allowed him to carry out a mathematical analysis of the data gathered by Geiger and Marsden.
Working with alpha radiation
Alpha particles from a radioactive source were allowed to strike a thin gold foil. Alpha particles produce a tiny, but visible flash of light when they strike a fluorescent screen.
Surprisingly, alpha particles were found at large deflection angles and some were even found to be back-scattered.
Alpha and beta radiations were identified by Rutherford in 1899. Rutherford and Royds showed that an alpha particle was a helium-4 nucleus in 1909.
Rutherford knew that alpha radiation had a range of about 5 cm in air, and its range in denser materials had been measured.
Rutherford's scattering experiment:
1. Most of the fast moving α-particles passed straight through the gold foil.
2. Some of the α-particles were deflected by the foil by small angles.
3. Surprisingly one out of every 12,000 alpha particles appeared to rebound.
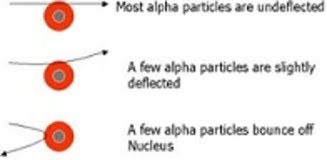
Conclusion of Rutherford's scattering experiment and observation:
1. Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
2. Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
3. A very small fraction of α-particles were deflected by very large angles, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
From the data he also calculated that the radius of the nucleus is about 105 times less than the radius of the atom.
Limitations of Rutherford Atomic Model
Rutherford atomic model was based on experimental observations it failed to explain certain things. Rutherford proposed that the electrons revolve around the nucleus in fixed paths or orbits. As per the Rutherford model, an electron would collapse in the nucleus in less than 10-8 seconds.
therefore Rutherford model was not in accordance with Maxwell’s theory and could not explain the stability of an atom.
The drawbacks of the Rutherford model was that he did not say anything about the arrangement of electrons in an atom which made his theory incomplete.
Although early atomic models were also inaccurate and failed to explain certain experimental results and became the base for future developments in the world of quantum mechanics.
Similarities and differences between Thomson's and Rutherford's atomic models
The similarities between Thomson and Rutherford's atomic model is :
There are negative and positive charges in an atom.
There is similarity is in shape - Thomson describes a sphere shape and Rutherford describes orbiting around the nucleus that is also sphere shaped.
The difference between Thomson and Rutherford model of atom is that Thomson model of atom does not contain any details about nucleus but Rutherford model of atom describe about the nucleus of an atom.
Sir, J.J. Thomson was the first to discover the subatomic particle called electron in 1904.
The similarities and differences between Bohr atomic model and Rutherford atomic model
In Bohr atomic model, electrons orbited the nucleus in quantised orbits. Bohr built upon Rutherford's model of the atom.
In Rutherford's model most of the atom's mass is concentrated into the centre (nucleus) and electrons are surround the positive mass in something like a cloud.
