Plants water relations
The water is important in plants physiological activities and in all living organisms. It provides the medium in which most substances are dissolved.
The protoplasm of the cells is nothing but water in which different molecules are dissolved and suspended. A watermelon has over 92% water, most herbaceous plants have only about 10-15% of its fresh weight as dry matter.
The distribution of water within a plant varies as woody parts have relatively very little water while soft parts mostly contain water.
A seed that may appear as dry but it still has water otherwise it would not be alive and respiring.
The terrestrial plants take up huge amount water daily but most of it is lost to the air through evaporation through the leaves (transpiration).
A mature corn plant absorbs almost three litres of water in a day while a mustard plant absorbs water equal to its own weight in about 5 hours.
The water potential
There are certain standard terms are necessary to comprehend plant-water relations as follows:
Water potential (Ψw)
Solute potential (Ψs) and
Pressure potential (Ψp)
Solute potential and pressure potential are the two main components that determine water potential.
The water molecules possess kinetic energy. The higher concentration of water in a system, higher is its kinetic energy or say water potential.
Therefore it is obvious that pure water will have the greatest water potential.
If two systems containing water are in contact, random movement of water molecules will result in net movement of water molecules from the system with higher energy to the one with lower energy.
Thus water will move from the system containing water at higher water potential to the one having low water potential. This process of movement of substances down a gradient of free energy is called diffusion.
If some solute is dissolved in pure water the solution has fewer free water molecules and the concentration (free energy) of water decreases, reducing its water potential.
Hence, all solutions have a lower water potential than pure water, the magnitude of this lowering due to dissolution of a solute is called solute potential or Ψs, it is always negative.
The more the solute molecules, the lower (more negative) is the Ψs.
The solution at atmospheric pressure, the water potential is equal to solute potential means Ψw = Ψs.
If a pressure greater than atmospheric pressure is applied to pure water or to a solution, its water potential increases.
It is equivalent to pumping water from one place to another.
Pressure can build up in a plant system when water enters a plant cell due to diffusion causing a pressure built up against the cell wall, it makes the cell turgid this increases the pressure potential.
Pressure potential is usually positive, though in plants negative potential or tension in the water column in the xylem plays a major role in water transport up a stem.
The Water potential of a cell is affected by both solute potential and pressure potential. The relationship between them is as follows:
Ψw = Ψs + Ψp
Osmosis
The plant cell is surrounded by a cell membrane and a cell wall. The cell wall is freely permeable to water and substances in solution hence is not a barrier to movement.
In plants the cells usually contain a large central vacuole, whose contents, the vacuolar sap, contribute to the solute potential of the cell.
In plant cells, the cell membrane and the membrane of the vacuole, the tonoplast together are important determinants of movement of molecules in or out of the cell.
Osmosis is the term used to refer specifically to the diffusion of water across a differentially or selectively permeable membrane.
The osmosis occurs spontaneously in response to a driving force. The net direction and rate of osmosis depends on both the pressure gradient and concentration gradient.
Water will move from its region of higher chemical potential (or concentration) to its region of lower chemical potential until equilibrium is reached.
At equilibrium the two region should have nearly the same water potential.
Osmotic solutions
There are different types of solutions as follows:
1. Isotonic solution: It is one that has the same concentration of solutes both inside and outside the cell.
2. Hypertonic solution: It is one that has a higher solute concentration outside the cell than inside.
3. Hypotonic solution: It is the one that has a higher solute concentration inside the cell than outside.
Types of osmosis
There are two types of osmosis as follows:
1. Endosmosis: The osmosis is known as endosmosis, when a substance is placed in a hypotonic solution, the solvent molecules move inside the cell and the cell becomes turgid or undergoes deplasmolysis.
2. Exosmosis: The osmosis is known as exosmosis, when a substance is placed in a hypertonic solution, the solvent molecules move outside the cell and the cell becomes flaccid or undergoes plasmolysis.
Difference between osmosis and diffusion
| Osmosis | Diffusion |
|---|---|
| Limited to the liquid medium only. | Occurs in liquid, gas and even solids. |
| Requires a semipermeable membrane. | Does not require a semipermeable membrane. |
| Depends on the number of solute particles dissolved in the solvent. | Depends on the presence of other particles. |
| Needs water for the movement of particles. | Does not need water for the movement of particles. |
| Only the solvent molecules can diffuse. | Both the molecules of solute and solvent can diffuse. |
| Particles flow occurs only in one direction. | Particles flow occurs in all the directions. |
| Additional pressure on the solution side may stop or reverse the entire process. | This process neither be stopped nor reversed. |
| Occurs only between similar types of solutions. | Occurs between the similar and dissimilar types of solutions. |
| It involves the movement of only solvent molecules from one side to the other side. | It involves the movement of all the particles from one region to the other region. |
| The concentration of the solvent does not become equal on both sides of the membrane. | The concentration of the diffusion substance equalizes to fill the available space. |
| Depends on solute potential. | Does not depend on solute potential, pressure potential, or water potential. |
| Only water or another solvent moves from a region of high energy or concentration to a region of lower energy or concentration. | Any type of substance moves from area of highest energy or concentration to region of lowest energy or concentration. |
| Not associated with uptake of minerals and nutrients. | It helps in the uptake of minerals and nutrients. |
Plasmolysis
The behaviour of the plant cells or tissues with regard to water movement depends on the surrounding solution. It may be isotonic, hypotonic or hypotonic. Plasmolysis occurs when water moves out of the cell and the cell membrane of a plant cell shrinks away from its cell wall.
This occurs whenthe cell or tissue is placed in a solution that is hypertonic (has more solutes) to the protoplasm. Water moves out, it is first lost from the cytoplasm and then from the vacuole.
The water when drawn out of the cell through diffusion into the extracellular (outside cell) fluid causes the protoplast to shrink away from the walls. The cell is said to be plasmolysed.
The movement of water occurred across the membrane moving from an area of high water potential (the cell) to an area of lower water potential outside the cell.
What occupies the space between the cell wall and the shrunken protoplast in the plasmolysed cell?
When the cell (or tissue) is placed in an isotonic solution, there is no net flow of water towards the inside or outside. If the external solution balances the osmotic pressure of the cytoplasm it is said to be isotonic.
When water flows into the cell and out of the cell and are in equilibrium, the cells are said to be flaccid.
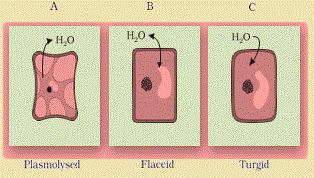
The process of plasmolysis is usually reversible.
When the cells are placed in a hypotonic solution (higher water potential or dilute solution as compared to the cytoplasm), water diffuses into the cell causing the cytoplasm to build up a pressure against the wall, that is called turgor pressure.
The pressure exerted by the protoplasts due to entry of water against the rigid walls is called pressure potential Ψp.
Because of the rigidity of the cell wall, the cell does not rupture. This turgor pressure is ultimately responsible for enlargement and extension growth of cells.
Imbibition
Imbibition is a special type of diffusion when water is absorbed by solids – colloids – causing them to increase in volume. The classical examples of imbibition are absorption of water by seeds and dry wood.
The pressure produced by the swelling of wood was used by prehistoric man to split rocks and boulders.
If it were not for the pressure due to imbibition, seedlings would not have been able to emerge out of the soil into the open, they might not have been able to establish.
Imbibition is also diffusion since water movement is along a concentration gradient, the seeds and other such materials have almost no water hence they absorb water easily.
Water potential gradient between the absorbent and the liquid imbibed is essential for imbibition. In addition, for any substance to imbibe any liquid, affinity between the adsorbant and the liquid is also a pre-requisite.
